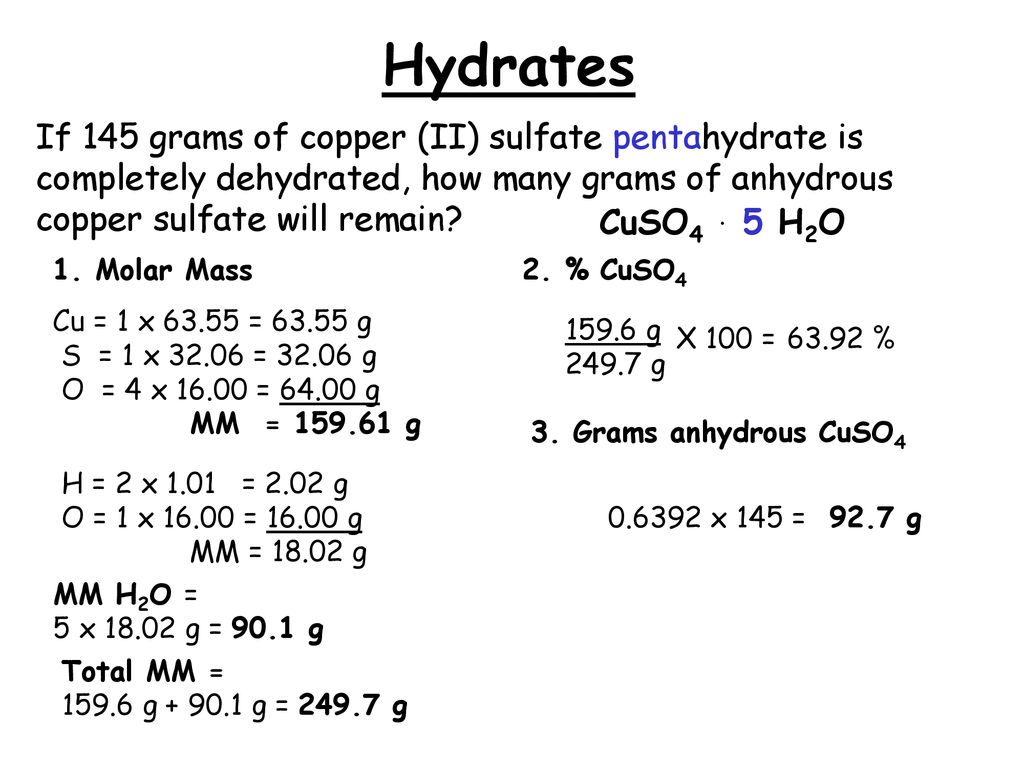
Molar Mass of Hydrated Copper Sulfate
When its heated the crystals crumble and turn white. Mass of sulphur 32.

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube
_____g CuSO 4 Determine the mass of water lost from Hydrate.

. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Using the mass of the copper II sulfate hydrate and the mass of the copper II sulfate anhydrate determine the mass of water lost from the hydrate. Mass of anhydrous copperII sulfate copied from yesterdays data sheet_____ 3.
Mass of hydrogen 1. It contains a copper II sulfate. Cuso45h2o 6353241651216 6353264518 2495g.
Anhydrous Copper II Sulphate CuSO4 This is less common to come across it is a white powdery solidand can be obtained by heating the pentahydrated form. It is a hydrate and a metal sulfate. Determine the correct formula and name of the hydrate.
N mMM 353 g 1596 gmol 002212 moles. As the molar mass of copper sulfate pentahydrate is 2497 gmol the amount of moles to start was. A mole of CuSO45H2O contains 5 moles of water which corresponds to 90 grams of water as part of its structure.
Anhydrous copper sulfate 591g. The rules of engagement roe working group is often used to. The molar mass of water is 18015 gmol and the molar mass of anhydrous copper sulfate is 159609 gmol.
Mass of clean dry test tube. After heating the CuSO4 that remains has a mass of 254 g. With your hydrated copper sulfate.
Which of the above hydrates do you think is. A sample of copper II sulfate hydrate has a mass of 397 g. So mass of copper 635.
1 Get Other questions on the subject. 2545 g of hydrate is heated and 6385 of the residue is found after heating. INCLUDE UNITS EVERY STEP AND keep dp when or AND keep sig fig when x or.
Copper II sulfate pentahydrateA few things to consider when finding the molar mass for CuSO4. Molar mass of CuSO4 1596086 gmol. 1 Cu atom and 9 O atoms.
Note the amount of water being evaporated from the compound as it is being heated. Copper sulfate is a hydrated crystal with the formula CuSO45H20 and a deep blue color. 4 rows CopperII Sulfate molecular weight.
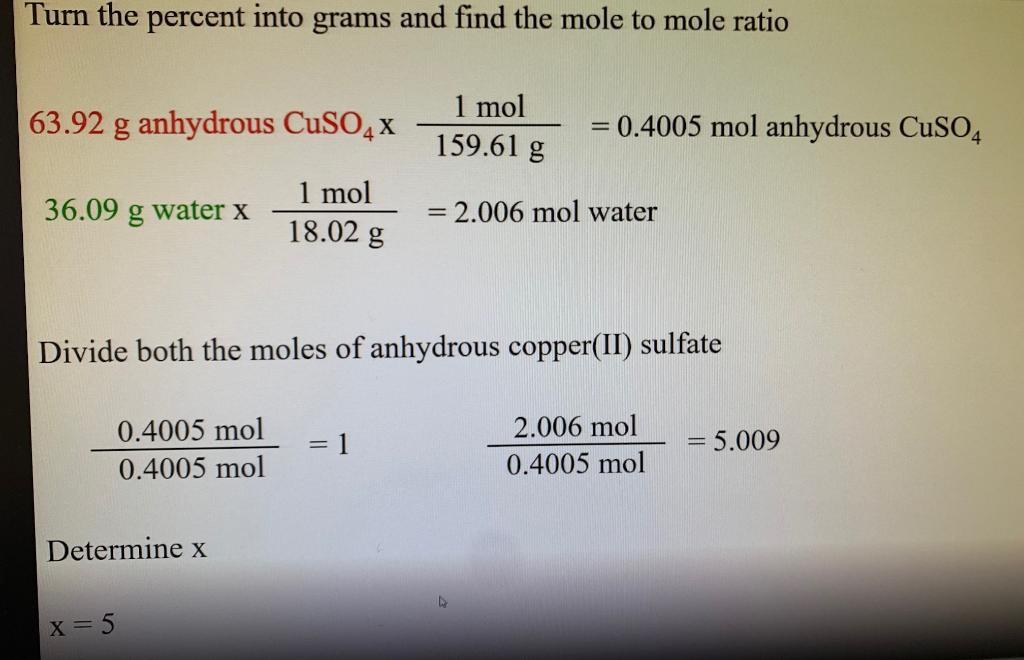
Explanation of how to find the molar mass of CuSO4 5H2O. A hydrate of copper II sulfate with formula CuSO4xH2O has a molar mass of 2500 gmol. My observation table looks like this.
Therefore the substance CuSO45H2O always consists of 90250 or 36 water by weight. A Determine the value of x in the formula of the compound. How many moles of water are in copper sulfate hydrate.
It is a potent emetic and is used as an antidote for poisoning by phosphorus. Hydrated copper sulfate 1000g. 216g Mass of.
The pentahydrate CuSO45H2O the most commonly encountered salt is bright blueFeb 17 2016. Science Chemistry QA Library Determining the formula of a hydrate. Copper ii sulfate heptahydrate.
Mass of hydrated copper sulfate in CuSo45H2O Mass of hydrated copper sulfate in CuSo45H2O Answers. Large crystals 1040 mm small crystals 210 mm snow crystals less than 2 mm and windswept powder less than. How many grams of oxygen are in this sample.
A sample of copper sulfate pentahydrate CuSO45H2O molar mass of CuSO45H2O 24968 gmol contains 3782g of Cu. Using the mass of the beaker and the mass of the beaker with copper II anhydrate determine the mass of the copper II sulfate anhydrate. Heat crucible with hydrate until all color has left the hydrate.
The molecular mass will be the sum of all masses of all atoms present. Per the video the next step is to get the molar mass of the anhydrous copper sulfate and determine the number of moles. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula CuSO4H2Ox where x can range from 0 to 5.
Copper II sulfate pentahydrate is the pentahydrate of copper 2 sulfate. As the molar mass of anhydrous copper sulfate is 1596 gmol the amount of moles of anhydrous copper sulfate is. N mMM 496 g 2497 gmol 001986 moles.
Four types of crystal size are provided based on its usage. A sulfate salt of copper. Copper Sulfates Water of Hydration Lab Calculation Sheet.
Mass of oxygen 16. 1 formula unit of CuSO45H2O contains. Chemistry 22062019 0400 miamassimino.
A bright blue crystalline solid. Find the mass of the unknown and crucible together. Mass of hydrated copperII sulfate copied from yesterdays data sheet_____ 2.
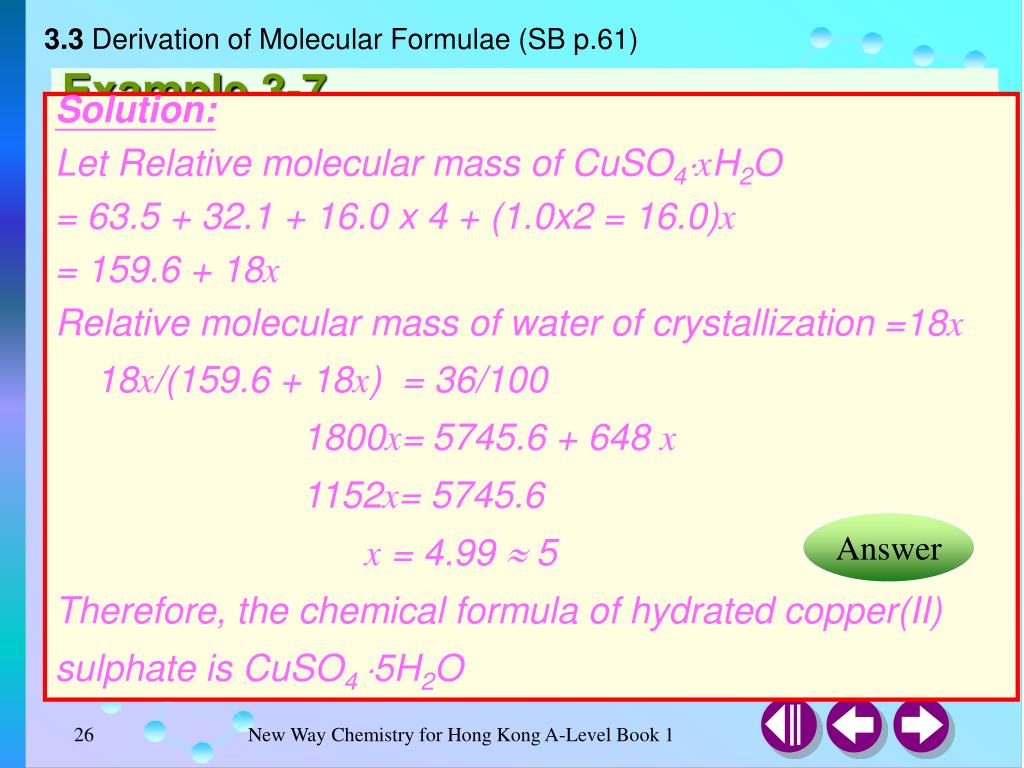
We had to find the molecular formula of a hydrate of copper 2 sulphate CuSO4 xH2O. Copper II sulphate heptahydrate. Formula of hydrated copper sulphate is.
Calculate the molar mass of each hydrate. How many moles of anhydrous copper sulfate do we have.

Molar Mass Molarity Molar Mass Mass In Grams Of One Mole Of An Element Or Compound Numerically Equal To The Atomic Weight Of The Element Or The Sum Ppt Download
Percent Composition Empirical Formulas Molecular Formulas Ppt Download

Determining The Molecular Formula Of A Hydrated Copper Sulfate Youtube

Calculate The Relative Molecular Mass Of Hydrated Copper Sulphate Cuso4 2h2o H 1 O 16 S 32 Cu 64 Experts Please Answer It Urgent Chemistry The Language Of Chemistry 14286463 Meritnation Com

Percent Composition Practice Problem 3 Cuso4 5h2o Youtube

To Find The Formula Of Hydrated Copper Ii Sulfate

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Solved Sample Data Mass Of Sample Hydrated Copper Ii Chegg Com
Molar Mass Molecular Weight Of Cuso4 Copper Ii Sulfate Youtube
Solved Sample Data Mass Of Sample Hydrated Copper Ii Chegg Com

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1

Chapter 10 The Mole The Mole What Do You Ask For When You Buy 2 Shoes 12 Eggs 48 Doughnuts 500 Sheets Of Paper 1 Pair 1 Dozen 4 Dozen 1 Ream Ppt Download

Molar Mass Of Cuso4 5h2o Youtube

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1
Ppt Chapter 3 Powerpoint Presentation Free Download Id 6008152

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

General Chemistry Copper Sulfate Lab

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1

Comments
Post a Comment